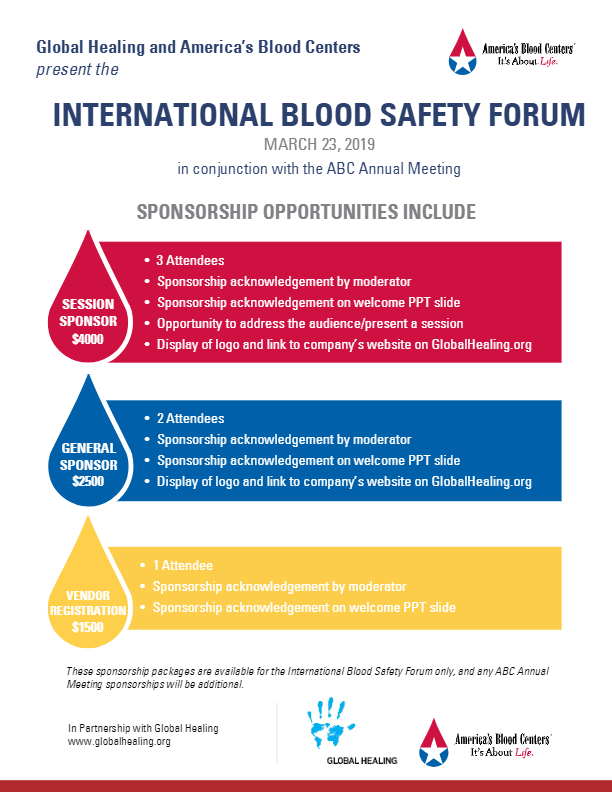
International Blood Safety Forum
Improving Access to Safe Blood: The International Blood Safety Forum
March 23, 2019 / Alexandria, VA
IBSF 2019 Survey
Jan Bult
Mr. Bult started his career in 1974 in the pharmaceutical industry. He has held various positions in sales, marketing, research and development, training and business management with CIBA-GEIGY, SCHERING AG, RHONE-POULENC and BIOTEST.
In 1995 he joined the European Association of the Plasma Products Industry as Executive Director before he was promoted to President & CEO of the Plasma Protein Therapeutics Association (PPTA) in January 1998.holds his current position as PPTA President since 1998. After his retirement from this position at the end of 2018 he became President Emeritus and continues to support the Association in 2019.
During his career he worked diligently to further enhance the capabilities of the plasma protein industry to provide lifesaving therapies to patients in the different parts of the world very seriously.. His activities took place in Asia, Europe and North America. In that role he was a frequent speaker at international conferences and worked with several patient groups. He is considered a well connected expert in the field of plasma protein therapies.
Mr. Bult is an FAA licensed pilot and enjoys flying a small aircraft. He and his wife Rose are the loving parents of five children and seven grandchildren.
Paula Castellanos Fernandez, QB, EIHBS, MA, MSc
Paula Castellanos is a Biochemical Chemist graduated at San Carlos University of Guatemala, with a Postgraduate degree in Immunohematology and Blood Banks by the same university, Postgraduate in Epidemiology, Postgraduate in Applied Immunohematology by Technological of Monterrey, Master in Industrial Administration and Service Companies at San Carlos University and Master in Transfusion Medicine and Advanced Cell Therapies by Autonomous University of Barcelona, Spain.
She is Professor of Biology and Mathematics at the Del Valle University of Guatemala
She has attended multiple congresses as a participant and lecturer. Exhibitor of various topics in Blood Bank and Transfusion Medicine in countries like Costa Rica, Colombia, Ecuador, Peru, Dominican Republic, Argentina, Chile, Mexico and Brazil.
She has been a professor at San Carlos University and Rafael Landívar University, Post-degree Coordinator in Immunohematology and Blood Banks of USAC and UNI. OPS distance tutor at the course Safe Blood Components for technical staff of the Blood Banks. Founder and teaching coordinator of the career of University Technician in Hemotherapy and Immunohematology at the Galileo University. Teacher at the Blood Bank Course at the ninth cycle of the Biological Chemistry Career at the Galileo University.
Adviser of several research projects in combination with the Faculty of Chemical Sciences and the Postgraduate School at Medicine Faculty in San Carlos University of Guatemala. She has conducted independent research projects including the use of Platelet Gel Rich in Platelet-derived Growth Factors in ulcerated and orthopedic patients of the General Trauma Hospital in the Guatemalan Social Security Institute and preparation of autologous collyrium in corneal ulcers for patients of Ophthalmology Department of the same hospital. She has several publications in scientific journals in Guatemala, Argentina, and Colombia.
She has participated in multiple commissions and associations, including Member of the National Commission of Blood Banks by the Guatemalan Social Security Institute, Vice President of the Ibero-American Cooperative Group on Transfusion Medicine (2011-2015), President of the Hemotherapy Association of Guatemala (2013-2017), President of ADonAS (Altruistic blood donors association of Guatemala) from 2017 until 2019, President of Ibero-American Cooperative Group on Transfusion Medicine (2017-2019).
Collaborator and writer of the Chapter “Platelet preparation, conservation and storage” in the book Applications and Practice of Transfusion Medicine edited by the G-CIAMT in 2013. Collaborator and writer in the Book of Management and Direction of Blood Banks with the chapters “Human Resources Management” and “Implementation of Information Systems” edited by G-CIAMT in 2015. Guatemalan representative in the EuroSociAL project for the promotion of voluntary donation in Latin America.
She currently works as Director of the Transfusion Medicine and Blood Bank Service of the General Trauma Hospital in the Guatemalan Social Security Institute, Teacher and Coordinator at the Postgraduate Program in Immunohematology and Blood Banks at the UNI 2014-2016. Teacher and Coordinator in the University Technician in Haemotherapy and Immunohematology at the Galileo University for the period 2012 until today. Teacher at the Blood Bank course for Biochemical Chemist at the Galileo University (2014 until today). INIADES Director from 2017.
Sushil G. Devare
Dr. Sushil Devare is a Distinguished Research Fellow and Director of Diagnostics Research and Emerging Markets Initiatives at the Abbott Laboratories, Abbott Park, Illinois, USA. Dr. Devare has directed and implemented research projects to identify and develop HIV, HTLV, HCV and HBV reagents/assay prototypes that are used in over 40 Abbott diagnostic and blood screening assays. He was member of the Abbott team that developed the world’s first HIV diagnostic test approved by the FDA in 1985. His research interests include: the implications of HIV variation on diagnosis and monitoring of viral infection, and emerging infectious diseases. Dr. Devare has authored 139 publications in scientific journals and has 22 issued U.S. patents to his credit.
Quentin Eichbaum, MD. PhD, MPH, MFA, JD, MMHC, FACP, FASCP
Quentin Eichbaum was born and raised in Namibia and South Africa. He initially studied law at the University of Cape Town and then completed his MD, MPH, PhD/postdoctoral studies at Harvard Medical School and the Massachusetts Institute of Technology in Boston followed by residency and fellowship training at Massachusetts General Hospital. He is currently Professor of Pathology, Microbiology and immunology and Professor of Medical Education and Administration at Vanderbilt University. He serves on numerous national and international pathology and global health committees and is on the CUGH Board of Directors, Chair of the CUGH Education Committee, and Co-chair of the AFREhealth-CUGH Working Group. He is Medical Co-Director of the Vanderbilt University Medical Center Transfusion Service (VUMC), Director of the Transfusion Medicine Fellowship Program, Director of the Vanderbilt Pathology Program in Global Health, and Medical Director of the VA Tennessee Valley Health Care System Blood Bank.
Jed B. Gorlin MD, MBA
Dr. Jed Gorlin is board certified in pediatrics and blood banking and transfusion medicine. Dr. Gorlin is the VP of Medical and Quality Affairs for Innovative Blood Resources and Medical Director, Community Blood Center of Greater Kansas City. His education includes a BS-Stanford, MD Yale, and MBA from U of MN Carlson School of Business.
He trained in Pediatrics and Pediatric Hematology/Oncology at Boston Children’s hospital, and completed research fellowships at Dana-Farber Cancer Institute, Mass. General, Brigham & Women’s Hospital, and the Puget Sound Blood Center. He is associate clinical professor of laboratory medicine at University of Minnesota.
He is currently the co-director of Transfusion Medicine at Hennepin County Medical Center, a level one trauma hospital in Minneapolis, and Children’s Hospitals and Clinics in Minneapolis and St. Paul, MN. He is a consultant to AABB, CDC and NIH for projects in Rwanda, Tanzania and Central Asia including Kazakhstan and Kyrgyzstan. He was the AABB liaison to ACOG, AWHONN and CMQCC for obstetric hemorrhage and ABC liaison to AABB TTD committee. He makes great biscotti and pignoli cookies.
CDR Jonathan Hoiles, MBA, MS, MT(ASCP) SBBCM, CQA(ASQ) Medical Service Corps, U.S. Navy
CDR Jonathan Hoiles received his Bachelor of Science in Medical Technology from the University of South Alabama in 1998. Upon graduation he worked at the University of Alabama at Birmingham (UAB) Medical Center as a staff Medical Technologist. In 2000 he received a commission in the U.S. Navy and was assigned to Naval Medical Center San Diego where he worked in all areas of the Laboratory Department. In 2003 he transferred to Naval Hospital Keflavik, Iceland where he served as the Laboratory Department Head.
In 2005, CDR Hoiles was selected to the Armed Services Blood Bank Fellowship at Walter Reed Army Medical Center. Upon graduation from the fellowship and earning a Master of Science degree in Immunohematology from the George Washington University, CDR Hoiles returned to Naval Medical Center San Diego to serve as the Division Officer in charge of the Blood Donor Center. In 2008, he volunteered for a year-long deployment to Afghanistan where he served as an advisor to the Afghan National Army. In 2009 he reported for duty at Naval Hospital Camp Lejeune, North Carolina. For the next two years he served as the Department Head of the Blood Donor Center and the Division Officer for Transfusion Services. In 2011 he reported to Naval Hospital Okinawa, Japan where he was assigned as the Director of the U.S. Pacific Command Armed Services Blood Bank Center. In 2014 he reported to Walter Reed National Military Medical Center in Bethesda, Maryland to serve as the Chief of Blood Services with the responsibilities for managing the Transfusion Service, Apheresis Center, Armed Services Blood Bank Center, and the satellite blood collection center at the Pentagon. Additionally, he began working with the President’s Emergency Plan for AIDS Relief (PEPFAR) program to improve blood safety in Ethiopia.
In 2016 CDR Hoiles reported to the Bureau of Medicine and Surgery to serve as the Director, Navy Blood Program. As the Director, he has overall responsibility for coordination and management of all Navy Blood Program matters for the Surgeon General of the U.S. Navy.
CDR Hoiles has been awarded the Defense Meritorious Service Medal, Meritorious Service Medal, four Navy and Marine Corps Commendation Medals, two Navy Marine Corps Achievement Medals, Army Achievement Medal, and various other unit and campaign awards.
Jerry A Holmberg
Dr. Jerry Holmberg is the Senior Director of Strategic Scientific Innovations at Grifols Diagnostic Solutions Inc. where he provides scientific direction to Grifols’ business opportunities in advancing blood safety through the entire transfusion chain. He has more than 35 years in all areas of laboratory medicine with a concentration on blood bank operations, education, and policy. Dr. Holmberg served twenty years in the US Navy, achieving the rank of Commander. During his military career he held various positions directing blood banks, blood donor centers, frozen blood bank depots, blood bank computer systems and policy development within the Navy Blood Program. In 2014, CDR Holmberg (Ret) received the Armed Services Blood Program’s Lifetime Achievement Award from the Department of Defense.
For over eight years, Dr. Holmberg served his nation again in public health as the Senior Advisor for Blood Policy for the Secretary of Health within the Department of Health and Human Services (HSS) and Executive Secretary for the Advisory Committee on Blood Safety and Availability. During his tenure as a public servant, he served three Secretaries of HHS and three Assistant Secretaries for Health. He also led the Department in a review of the blood donor deferral for men who have had sex with other men (MSM) policy, pathogen reduction technology, Hepatitis Action Plan, biovigilance, blood inventory management and disaster planning. He was also an expert advisor to the World Health Organization during the years 2003-2011 . In 2009, Dr. Holmberg received the AABB President’s award for his activities in biovigilance.
His concern and dedication to serve the underserved has motivated him to actively participate in the President’s Emergency Plan for AIDS Relief (PEPFAR) from 2004-2011 . Eliminating the risk of transfusion transmitted diseases, especially HIV, in the blood supply has been a personal goal. While the activities of PEPFAR were primarily located in Africa and Central Asia Republics, over the last six years, he has traveled to many countries in Asia and South America to provide technical assistance in blood safety and availability. Since 2013 he has been involved with the Asia Pacific Economic Cooperative (APEC) through the Life Science Innovation Forum to raise the awareness of laboratory quality and Good Manufacturing Practices.
Dr. Holmberg received his B.S. in Biology from Saginaw Valley State College (now Saginaw Valley University), his Medical Technology training at St. Luke’s Hospital in Saginaw, Michigan and M.S. in the Department of Pathology, Clinical Laboratory Science from Michigan State University. A Fellowship in Blood Bank at Walter Reed Army Medical Center led to certification as a blood bank specialist. Following his Fellowship, he obtained a Ph.D. in Biological Sciences from Bowling Green State University (Ohio) with an emphasis on immunohematolgy. He holds certifications in medical technology, specialist in blood bank technology, and quality assurance.
Dr. W. Martin Smid
W. Martin Smid (1958, Netherlands, MD Medicine, PhD and MBA) started his medical career in 1988 as MD Internal Medicine for 3 years and 5 years as Consultant for the Coagulation in the University Hospital Groningen. In 1996 he moved to the blood bank, later Sanquin, where he had a variety of management functions mainly with medical responsibilities including stem cell collection. Since 2005 he is Managing Director of Sanquin Consulting Services, a unit that facilitates knowledge transfer in transfusion medicine by collaborations with blood services, society and the health care sector. His main responsibilities are international cooperation and knowledge sharing in international projects. As director of the Academic Institute for the International Development of Transfusion Medicine (JDTM), he is responsible for the MTM programme at the Graduate School of Medical Sciences of the University of Groningen. Martin Smid is a member of ISBT WP Global Blood Safety from the start in 2010 and since 2018 acts as Chair. He is involved in the WHO Global Blood Safety Network and AfSBT Educational Committee and advisor of the Curacao blood bank. Dr Smid also takes part in the WHO Global Blood Safety Network and participated in WHO activities. He is also international inspector for JAC1E accreditation for collection. Dr. Smid has over 40 international publications in transfusion, haemophilia and coagulation.
Dr. Paul Strengers
Paul Strengers obtained his Medical Degree from the University of Leiden, The Netherlands. He started in organ transplantation at the Academic Medical Centre in Amsterdam. In 1986, he joined the Central Laboratory of the Netherlands Red Cross Blood Transfusion Service (CLB). In 1992, a post-graduate course in Transfusion Medicine was followed in Edinburgh, Scotland, and in Clinical Epidemiology at Leiden University. He was Medical Director of CLB Blood Bank (1999-2003), Medical Director at CAF-DCF in Brussels, Belgium (2000-2017), Director Medical Affairs and Product Development at Sanquin Blood Supply, Division of Plasma Products, in Amsterdam (2003-2015), and Advisor to the Board of Sanquin Plasma Products (2015-2017).
His research and activities in blood transfusion medicine are focused on clinical transfusion medicine and sciences i.a. haemophilia and inhibitors to factor VIII, coagulation and the reversal of anti-coagulant therapy, intravenous immunoglobulins and its indications, haemovigilance and pharmacovigilance, and blood transfusion quality, ethics and organization.
Currently, he is an independent consultant, specialized in blood and plasma. In this capacity, he is Executive Director of the International Plasma and Fractionation Association on a freelance basis. Further he is a Member of the Expert Committee on Biological Standardization, section Blood, of the World Health Organization and Chairman of the – Supervisory Board of the Netherlands’ Royal Tropical Institute (KIT); Fellow at the Faculty of Pharmaceutical Medicine of the Royal Colleges of Physicians, UK; and member of international advisory boards, and international editorial boards of a number of scientific journals. He was President of the International Haemovigilance Network (2000-2006), Secretary-General of the International Society of Blood Transfusion (2000-2010), President of the International Plasma Fractionation Association (2012-2016) and Chairman of the Netherlands Red Cross, department of Amsterdam-Amstelland (2008-2018). He is Honorary Member of the International Society of Blood Transfusion and Corresponding Member of the Deutschen Geselischaft Mr Transfusionsmedizin und Immunhamatologie (DGTI). Publications include author or co-author of more than 150 scientific papers.
Nigel Talboys
Nigel has global responsibility for Government Affairs and Public Policy for Terumo BCT. He has a wide experience in the Medical Technology field and has worked for a number of multinational companies in a variety of business functions. These companies include Becton Dickinson and C.R. Bard.
During his time in Becton Dickinson he was involved in the development of new techniques used in regional anesthesia, particularly in the creating a market for Combined Spinal and Epidural Anesthesia. In C.R. Bard he developed a new business model in Europe used to treat prostate cancer with brachytherapy.
He has also been involved in several acquisitions and integration projects.
He is Chairperson of the Public Affairs Group, representing Industry at the European Trade Association (MedTech Europe) a board member of MedPharmPlast, Chairperson of the regulatory committee for MedPharmPlast, a board member of the UK / Japan 21st Century Group. The primary goal of the UK / Japan 21st Century Group is to serve as a catalyst for increasing the level of mutual understanding and awareness of the political, economic, and social environments in each country.
A British national, Nigel has spent the last 27 years working outside his home country in France, German and now Belgium. He studied Human Biology at Loughborough University in England and attended an Executive Business Program at Harvard University in Boston, USA.
For more information, please contact John Donnelly: john@globalhealing.org